Difference between ideal fuel cell and actual fuel
cell is given below:
Actual fuel cell
|
Ideal fuel cell
|
In actual fuel cell
the transport of oxidizer electrolyte fuel and product from electrode.
|
Proper proportion and
maintained.
|
And Surface is not
proper proportion and complex.
|
System is simple.
|
Homogeneous reaction
turbine place for three face of oxidizer a liquid fuel electrode and solid
electrode.
|
No homogeneous
reaction takes place.
|
The diffusion process
at the electrode surface is quiet complex.
|
In ideal fuel cell
the diffusion process at the electrode surface is quiet simple.
|
Too much liquid or
gas enters the electrolyte and reduce the surface.
|
No liquid gas enters.
|
In an actual fuel
cell activation energy reduces the work of cell.
|
In ideal fuel cell
there have no activation energy.
|
In an actual fuel
cell spurious chemical and electrochemical reaction takes place at various
places within the cell.
|
In ideal fuel cell
this reaction is absence.
|
In an actual fuel
cell need some extra energy for various auxiliaries such as pumps, fans and coolant
system.
|
There are not
necessary in ideal fuel cell.
|
In an actual fuel
cell there have some consideration such as
i.
Initial cost.
ii.
Environment effect.
iii.
Safety.
iv.
Reliability.
v.
Metal should be corrosive.
|
In an ideal fuel
there have no consideration.
|
Ohomic resistance
reduces the terminal electromotive force and work of the cell.
|
There have no Ohomic
resistance.
|
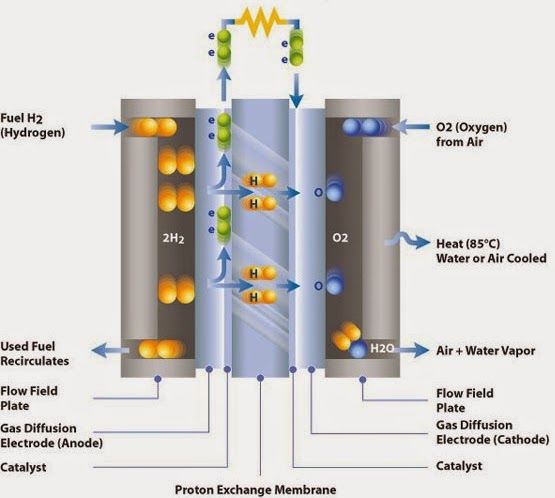
Figure 1: Hydrogen and Oxygen Fuel cell
Figure 2 : Hydrogen and Oxygen Fuel cell

Comments
Post a Comment